In a state in which less than half of the population is vaccinated, April Karli could only watch with disdain as ICU units in Austin, Texas, swelled with patients. COVID-19 cases had risen by 92 percent in a week — Karli's anti-vaxxer brother was among them. Her oldest daughter, who was inoculated with a Johnson & Johnson vaccine in March, was due to start her sophomore year of college — in person — in a matter of weeks. Her brother's illness, the new threat of breakthrough infections, and the efficacy of the J&J vaccine against the delta variant stoked Karli's concern for her daughter's safety. Fearful, Karli turned to Facebook for advice.
"Does anyone know if a state database will prevent someone who got J&J from self-boosting with Pfizer?" Karli wrote. "With delta and the high spread here in Texas, I'd like her to get her boosted before she goes back to her college campus."
As Israel, Germany, and the UK approved COVID-19 booster shots for the elderly and those with underlying conditions, and now that San Francisco is permitting those with a J&J vaccine to get an mRNA vaccine as a booster, too, many Americans are contemplating whether they might also need a COVID-19 vaccine booster shot. For those living in communities with significant outbreaks, the fear is heightened. And some intrepid citizens, like Karli, are attempting to double-vaccinate themselves and their loved ones, often skirting state public health rules regarding vaccines to do so.
"We have the second-highest number of cases in the country, and people here still don't believe COVID is real," Miami resident German Gobel said. "I don't see how I have much of a choice."
Karli and Gobel have quite a bit in common in this regard: both live in delta variant hotspots; both were participants in the Moderna COVID-19 vaccine trials, and both are members of a Facebook group by and for members of this unique demographic. The majority of posts on "The Page" this month, the group whose official name has been redacted out of privacy and safety concerns, discuss one topic: "self-boosting" — as it is known — the idea of self-administering booster shots by seeking out secondary or in some cases tertiary vaccinations. And while some vaccine trial participants have been getting booster shots for months as part of official clinical trials, others seek them out clandestinely.
As most Americans were locked in their homes waiting for their first COVID-19 vaccine, former Pfizer trial participant Tod Lewis of Austin, Texas, was invited to join Pfizer's new COVID-19 booster trial in late March.
Lewis and others posted on The Page about their booster trial experiences; several in the group who were anxious to again be "human guinea pigs" — as Lewis referred to himself — called their trial site administrators. There were rumors that only the prior trial participants who were "compliant" and "easy to work with" were given the prerogative of being in the new study. While disgruntled trial site employees hung up on some callers, Los Angeles resident and former Pfizer trial participant, Louise, who used a pseudonym due to legal concerns about self-boosting, got a spot in a Pfizer booster trial the first time she called.
Most weren't as lucky as Louise. As 2021 trudged on, the bulk of members on The Page reluctantly accepted they wouldn't be participants in any booster trials, and thus, wouldn't get boosters soon — at least, not by going through any official channels. In late spring, as some gloated about their participation in the new booster vaccine trials, studies that claimed protein-based vaccines like J&J and AstraZeneca provided better protection against the novel coronavirus when boosted with an mRNA vaccine were posted on The Page. A 74-year-old Greenville, South Carolina resident, Nancy McFarlane, took notice.
"I knew AstraZeneca wasn't as effective in people over 60 because of the studies coming out of Europe," the former AstraZeneca trial participant, who was fully vaccinated in early January, said. "More people got vaccinated in South Carolina than I expected, but it's still nowhere near where it should be. The governor made it illegal for municipalities and schools to pass mask mandates, and people didn't wear them when it was mandated," McFarlane said. "There's no way I trust people to be honest and wear a mask if they're unvaccinated."
For these reasons, McFarlane said got an antibodies test in May. Members of The Page have long used COVID-19 antibody tests as a marker of whether their vaccines are still efficacious, often posting, and comparing their results, as Gobel explained.
"We've shared each other's antibody results for months now," Gobel said. "First to know if you've got the placebo, then to know the efficacy, then to know the half-life of the antibodies and how long they last."
(April Karli's Roche assay antibody test results, Aug. 3, 2021. Screenshot courtesy April Karli)
The preferred test among members of The Page is the Roche Anti-SARS-CoV-2 assay antibodies test, which can be purchased online from Labcorp. The antibody titer, or "score" — an example of which is pictured above — refers to the total number of COVID-19 antibodies found in a person's blood. A dataset referenced in an article authored by Dr. Tony Ho, an infectious disease expert at the University of Texas Health Science Center at San Antonio, Texas, suggested that 30 U/mL was the minimum antibody titer in which an immunocompromised person would have enough neutralizing antibodies to fight off a COVID-19 infection. But, Ho told Salon that the threshold is likely higher when considering the antibodies necessary to protect against the delta variant.
"Delta is a new phenomenon, and data gathering is ongoing as we speak," Ho said. "Hopefully, we will get a sense of reasonable titer levels of protection against the delta variant soon."
McFarlane said her test results, which reported an antibody titer of 48.4U/mL, were too modest for her to feel protected from COVID-19, especially if she was to be seeing her five and eight-year-old grandchildren. McFarlane self-boosted with Pfizer, and is currently in-between doses. After the two-week interval between doses concludes, her second dose of Pfizer will be her fourth COVID-19 vaccine in six months. Louise, who was fully inoculated in September, said her antibody test from July, which reported an antibody titer of 100U/mL — a noticeable decline from her antibody test results in February — left her no choice other than to call her trial site and inquire about joining the Pfizer booster-trial.
"I feel personally that everyone who's concerned should be going out and getting that test," Louise said. " If you're immunocompromised — in any way — you might not have the same level of antibodies you think you have," she said.
Date of Louise's Pfizer Phase 3 Vaccine Shots:
- First Dose of Pfizer: Aug., 11, 2020
- Second Dose of Pfizer: Sept., 1, 2020
Louise's Roche Antibody Test Results
- February: 450U/mL
- May:127U/mL
- July:100 U/ML
Studies asserting that AstraZeneca was ineffective against the beta and lambda variant influenced Justin Cherniak's decision to self-boost, as he was planning a trip to Chile over summer and Europe in the fall. Though the 34-year-old Bay Area resident canceled both trips, Cherniak still wanted to be as protected as possible against these variants.
Want more health and science stories in your inbox? Subscribe to Salon's weekly newsletter The Vulgar Scientist.
"Having various comorbidities, I was quite worried about catching COVID," the prior AstraZeneca trial participant, said. "That, combined with assorted studies showing the mix of AstraZeneca and Pfizer was safe and effective, vindicated my decision," Cherniak said.
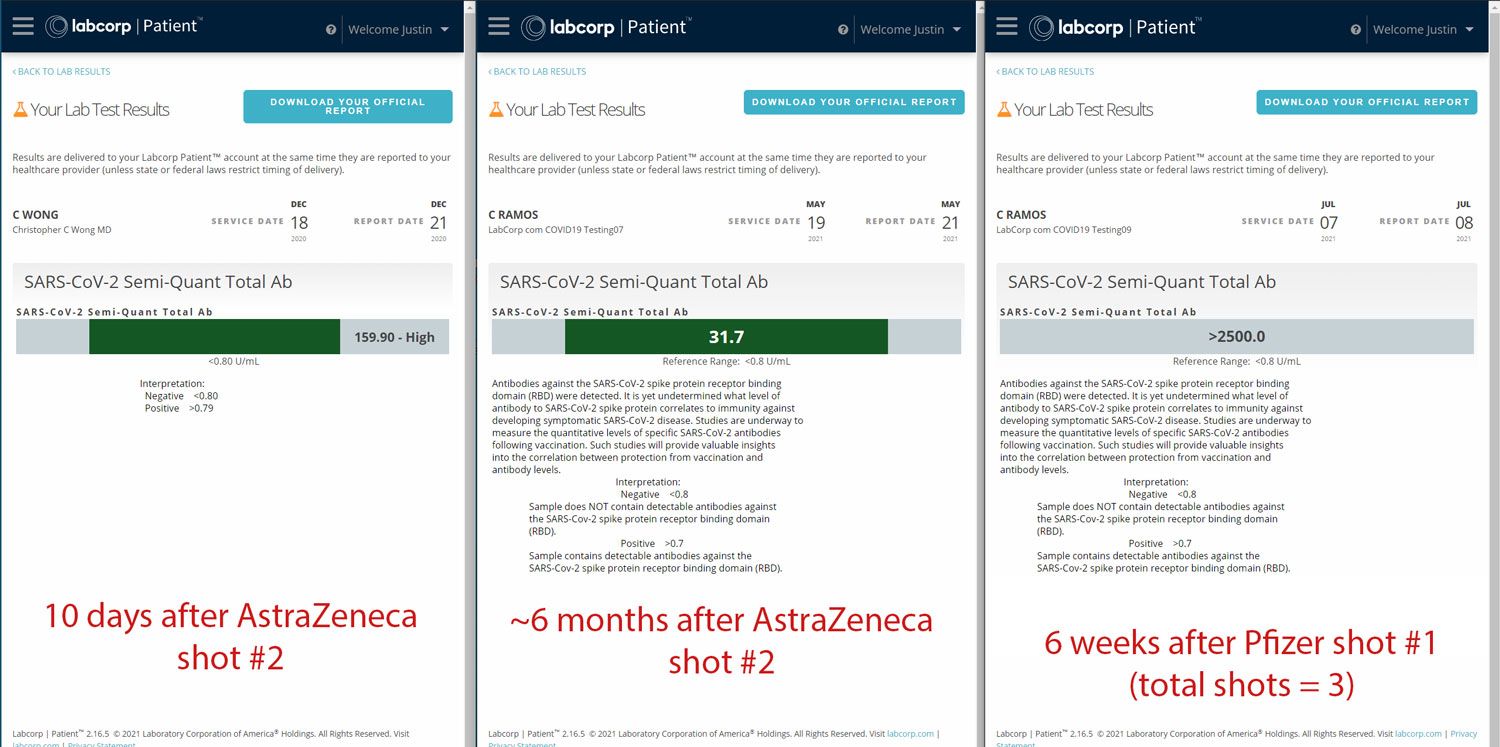
(Cherniak's Roche antibody test results from December, 2020, May, 2021, and July, 2021. Screenshot courtesy of Justin Cherniak.)
Both Louise and Cherniak were regularly testing their antibody titer, but only Cherniak reported his results to The Page before and after self-boosting. In his post to The Page, Cherniak included his antibody titer ten days — and six months — after complete inoculation via his second dose of AstraZeneca. He also reported his antibody test results six weeks after he self-boosted with Pfizer. Cherniak's self-administered mini-trial data detailed a significant decline of his antibody titer — 128.2U/mL — six months after his second dose of AstraZeneca. But, two months after self-boosting with Pfizer, his antibody titer was off the charts — reporting an antibody titer of 2500+ U/mL — a test result Cherniak cited as to why he decided against getting a second dose of the Pfizer vaccine.
Cherniak's data encouraged others like Gobel to get an antibody test and report their results before —and after — self-boosting. Although he is on a waitlist, hoping to join Moderna's vaccine booster trial, Gobel has decided that he will instead get an antibody test if he's not fortunate enough to be selected as a participant. And if his results report a titer of less than 400U/mL, he will self boost, most likely with two doses of Moderna — and he won't be alone.
"There's going to be four of us," Gobel said. "We're going to go in at the same time, take our test, compare results, and then make a decision. I'm pretty sure everyone's going to get a booster," he said.
Gobel and his colleagues plan to post their antibody test results —before and after self-boosting — on social media in an attempt to publicize the merits of boosters.
So many trial participants have posted their antibody test results to The Page that one member, who declined to be identified publicly, decided to keep track of the data. A retired professor with a Ph.D. in Cell Biology created and regularly updates a scatter plot correlating antibody levels with the number of days since trial participants received their second dose and, more recently, since boosters.
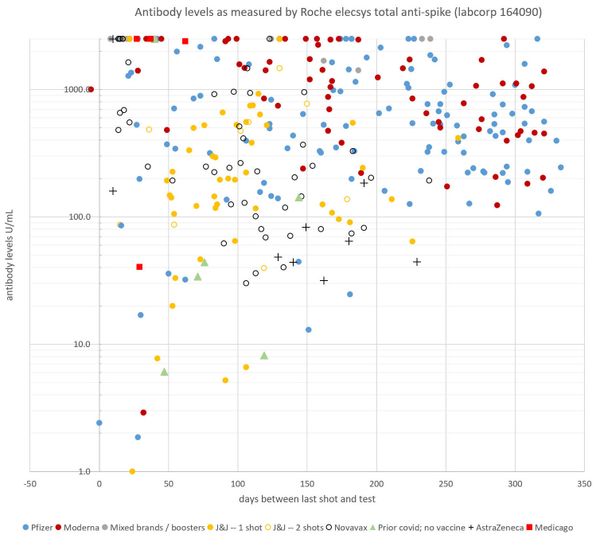
(Antibody data set collected and created by a pseudonymous member of a COVID-19 vaccine clinical trial Facebook group, who has a Ph.D in cell biology. The scatter plot comprises antibody tests for the Facebook group members.)
Pre/Post COVID-19 Booster Data reported by "The Page" members
First Vaccine: Antibody Titer (Before self-boosting) Booster Antibody Titer (post-self boosting)
2 Doses of Pfizer 1027U/mL Pfizer 2500+U/mL
1 Dose J&J 394U/mL Pfizer 2500+U/mL
2 Doses of Novavax 936U/mL Pfizer 2500+U/mL
2 Doses of Moderna 20U/mL Moderna 2500+U/mL
The antibody test result data reported by members of The Page correlate with studies that suggest vaccine efficacy wanes over time — a prospect that unsettles vaccine trial participants, who were amongst the first to be vaccinated for COVID-19. But, medical professionals like Dr. Monica Gandhi, a Physician, and Professor at the University of California at San Francisco, say that antibody titer results from the Roche antibody test should not determine whether self-boosting is necessary.
"There are two arms of the immune system, B-cells and T-cells," Gandhi said in an email to Salon. "B-cells produce antibodies, and T-cells protect you against severe infection. Measuring antibodies alone doesn't provide a full understanding of your level of protection, and it is generally agreed upon that the neutralizing antibody titer matters more," She wrote.
Although the Centers for Disease Control and Prevention (CDC) agrees with Dr. Gandhi, Dr. Ho does not.
"The Roche antibody test looks at antibodies specific to the receptor-binding domain for the spike protein," Ho said. "You have a higher chance of capturing a reasonable estimate of neutralizing antibodies because of this."
Several studies comparing the Roche antibody test to those only measuring neutralizing antibodies support Ho's claim. But, neither Gandhi, Ho, the CDC, nor the Food and Drug Administration (FDA) recommends or condone seeking a COVID-19 booster shot — unless a participant in one of the several booster trials.
According to Govind Persad, an expert in Health Law and Researcher at the University of Denver, self-boosting is also illegal. Since the three active vaccines received Emergency Use Authorization only, getting a booster is considered "unauthorized biologic."
"The FDA in principle could take action against a distributor, like a doctor or a pharmacist, who administers a booster, but this is unlikely," Persad said. "Lying to get a booster might be common-law or criminal fraud, but legal action is unlikely because the vaccine is free."
Though technically illegal, there are few barriers when it comes to getting an additional COVID-19 vaccine, as Karli and Louise discovered.
"I was going to test my daughter's antibodies first, but Austin started to surge with delta cases, and we had a good opportunity to get her a booster," said Karli, who herself decided against a self-boost after receiving test results that reported a high antibody titer of 338U/mL last week. "We just went to a different pharmacy, and she got a dose of Pfizer with no trouble."
Louise — who was enrolled in a Pfizer vaccine booster trial — lost her patience after the trial site called the night before she was due to get boosted, notifying her that the boosters had yet to arrive. Frustrated and panic-stricken, Louise walked into a RiteAid and got a dose of the Moderna vaccine — no questions asked.
"I guess I broke COVID vaccine law — but half of the country doesn't want to go out and freaking get it," Louise said. "My antibodies were so much lower than people who had gotten vaccinated more recently, and I really didn't feel like it was fair," she said. "Because I participated in this trial, I think that we should get boosted before everyone else."
The FDA — ignoring last week's recommendation from the World Health Organization that petitioned rich countries to not administer boosters until after the rest of the world had received at least one dose — authorized those who are immunocompromised to receive a third shot during a meeting today. The FDA's statement said, "Others who are fully vaccinated are adequately protected and do not need an additional dose of COVID-19 vaccine at this time."
"Frankly, I don't feel there's anything unethical because (the U.S) is literally wasting vaccines because people refuse to get vaccinated," Gobel said. "Now the CDC keeps flip-flopping, and some of us are kind of tired of it," he said. "There is data suggesting there are benefits to boosting, and there are humans on the other side of these trials."
Shares